Membrane-bound Alcohol Dehydrogenase And Aldehyde Dehydrogenase
They produce acetic acid from ethanol by two sequential catalytic reactions of membrane-bound alcohol dehydrogenase ADH and aldehyde dehydrogenase ALDH. Fatty aldehyde dehydrogenase or Long-chain-aldehyde dehydrogenase is an aldehyde dehydrogenase enzyme that in human is encoded in the ALDH3A2 gene on chromosome 17.

Fine Tuning Ethanol Oxidation Pathway Enzymes And Cofactor Pqq Coordinates The Conflict Between Fitness And Acetic Acid Production By Acetobacter Pasteurianus Gao 2021 Microbial Biotechnology Wiley Online Library
Request PDF Role of a membrane-bound aldehyde dehydrogenase complex AldFGH in acetic acid fermentation with Acetobacter pasteurianus SKU1108 Acetic acid fermentation is widely considered a.

Membrane-bound alcohol dehydrogenase and aldehyde dehydrogenase. Genetic variants in two enzymes involved in the metabolism of ethanol alcohol-dehydrogenase ADH1B 2 and aldehyde-dehydrogenase ALDH2 2 through increasing the blood level of acetaldehyde may play a protective role against alcoholism. Membrane-bound aldehyde dehydrogenase ALDH was purified from the membrane fraction of an industrial-vinegar-producing strain Acetobacter polyoxogenes sp. Function i Catalytic activity i.
Aldehyde dehydrogenase enzymes function to remove toxic aldehydes that are generated by the. Aldehyde dehydro- genase is solubilized from the membrane after alcohol dehydrogenase has been solubilized. Moreover the enzyme utilizes ethanol as a substrate in a reaction mechanism where this is subjected to a two-step oxidation.
The alcohol dehydrogenase ADH and the aldehyde dehydrogenase ALDH. In this study a new insight was provided to understand the functions of membrane-bound alcohol dehydrogenase mADH and aldehyde dehydrogenase mALDH in the bio-oxidation of primary alcohols diols and poly alcohols using the resting cells of Gluconobacter oxydans DSM 2003 and its mutant strains as catalyst. Enzyme activity of D-aldopentose 4-dehydrogenase aldehyde dehydrogenase ALDH and alcohol dehy-drogenase ADH was assayed with K-ferricyanide under the similar conditions described above using D-ribose acetaldehyde and ethanol used as substrate respectively.
Aldehyde Membrane-bound aldehyde dehydrogenase catalyzes the oxidation of aldehydes to corresponding acidsin vivo and the enzyme activity is linked to the electron transport system of the acetic acid bacteria. Diazotrophicus is indeed a double function enzyme which is able to use primary alcohols C2C6 and its respective aldehydes as alternate substrates. Oxydans 621H allowing the oxidation of a broad range of substances for energy generation.
Alcohol dehydrogenase of acetic acid bacteria acts on a wide range of primary alcohols except methanol. The purified enzyme was homogeneous on polyacrylamide disc gel electrophoresis PAGE. We present strong evidence showing that the well-known membrane-bound Alcohol dehydrogenase ADHa of Ga.
Here we used a markerless gene disruption method to construct a mutant of the Acetobacter pasteurianus stra. The membrane-bound alcohol dehydrogenase and the membrane-bound polyol dehydrogenase both with very broad substrate spectra seem to be most important enzymes in the membrane of G. Oxydans and catalyzes the oxidation of primary alcohols and diols to the corresponding acids or hydroxy acids Wei et al 2013.
To determine whether membrane-bound aldehyde dehydrogenase mALDH also plays a role in GA production in G. The first one is a pyrroloquinoline quinone PQQ-dependent membrane-bound alcohol dehydrogenase ADH and the second is a membrane-bound aldehyde dehydrogenase ALDH Matsushita et al. Gluconacetobacter polyoxogenes Acetobacter polyoxogenes Status.
Membrane-bound alcohol dehydrogenase ADH was purified from the membrane fraction of an industrial-vinegar-producing strain A. Gluconacetobacter diazotrophicus is a N2-fixing bacterium endophyte from sugar cane. The PQQ-containing membrane-bound alcohol dehydrogenase mADH is one of these key dehydrogenases in G.
Solubilization of Aldehyde Dehydrogenase. PNPLA3 I148M CG the transmembrane 6 superfamily member 2 TM6SF2 E167K and the membrane-bound O. Yakushi and Matsushita 2010.
To the membrane fraction 10 Brij 58 and 10 Tween 80 are added to a final concentration of 2 and 1 respectively. The enzyme acts as a vinegar producer by coupling with aldehyde dehydrogenase. The purified enzyme was homogeneos on polyacrylamide disc.
Download scientific diagram Affinity of ALDH to acetaldehyde in the membrane changes under fermentation conditions. From 55 substrates the alcohol dehydrogenase oxidized 30 substrates and the polyol. -Experimental evidence at protein level i.
Both these enzymes may reduce membrane ubiquinone and the reducing equivalents link to the respiratory chain to reduce molecular oxygen to. The results demonstrated that though both mADH and mALDH participated. Membrane-bound aldehyde dehydrogenase pyrroloquinoline-quinone Gene.
The three-component membrane-bound alcohol dehydrogenase ADH of Gluconobacter suboxydans IFO12528 was purified and the NH2-terminal amino acid sequence of each subunit was determined. The activities of other major membrane-bound dehydrogenases were also increased to some extent particularly membrane-bound aldehyde dehydrogenase mALDH which is involved in the catalytic oxidation of aldehydes to the corresponding acids and was 126-fold higher. The three-component membrane-bound alcohol dehydrogenase ADH of Gluconobacter suboxydans IFO12528 was purified and the NH2-terminal amino acid sequence of each subunit was determined.
A quinone an aldehyde H 2 O a carboxylate a quinol H EC. Acetaldehydeferricyanide oxidoreductase activity with different. Aldehyde is oxidized in vitro in the presence of dye and the rate of oxidation can be followed by the reduction of the dye.
NBI1028 by solubilization with Triton X-100 and sodium N-lauroyl sarcosinate and subsequent column chromatography on DEAE-Sepharose CL-6B and hydroxyapatite. NBI1028 by solubilization using Triton X-100 and subsequent column chromatography on DEAE-Sepharose CL-6B and hydroxyapatite. Aldehydes with a carbon ALPF Medical Research.
Products in the culture medium by using membrane-bound dehydrogenases localized on the outer surface of the cytoplasmic membrane12 Such incomplete oxi-dation is called oxidative fermentation and is carried out by membrane-bound enzymes like alcohol dehydrogen-ase ADH and aldehyde dehydrogenase ALDH in vinegar fermentation. The enzyme is localized on the outer surface of the cytoplasmic membrane and the oxidation of substrate is linked to its respiratory chain. Gluconobacter oxydans IFO12528 is able to produce glyceric acid GA from glycerol through the action of a membrane-bound alcohol dehydrogenase mADH which is required for GA production.
The oxidation of ethanol to acetic acid of this organism takes place in the periplasmic space and this reaction is catalyzed by two membrane-bound enzymes complexes. Europe PMC is an archive of life sciences journal literature. Acetic acid fermentation is widely considered a consequence of ethanol oxidation by two membrane-bound enzymes-alcohol dehydrogenase and aldehyde dehydrogenase ALDH-of acetic acid bacteria.
Membrane-bound aldehyde dehydrogenase ALDH in acetic acid bacteria acts on a wide range of aliphatic aldehydes except for formaldehyde. On the basis of the amino acid sequences the genes adhA encoding.
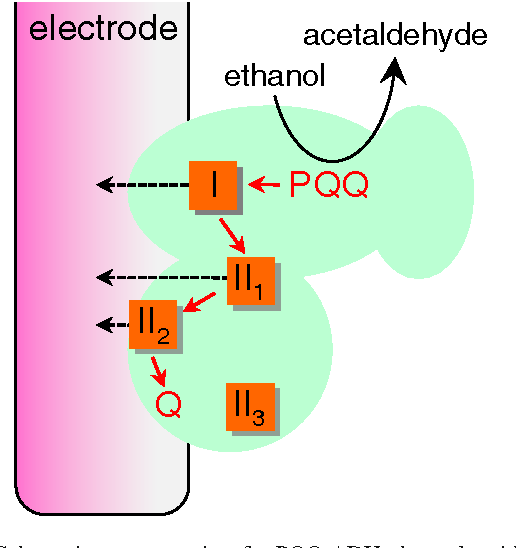
Alcohol Dehydrogenase Of Acetic Acid Bacteria Structure Mode Of Action And Applications In Biotechnology Semantic Scholar
Comments
Post a Comment